Abstract
Introduction : Hematopoietic stem cell transplantation (HSCT) has been applied as the curative treatment for rare pediatric non-malignant diseases. However, these patients are at risk of transplant related morbidity and there are no established conditioning regimens, especially for children without matched sibling donors. We evaluate the outcome of 43 pediatric cases with rare non-malignant diseases treated with HSCT.
Method : We performed a retrospective analysis of a total of 43 transplantations performed in 41 patients with rare non-malignant diseases between May 2002 and May 2017. The conditioning regimens were myeloablative (busulfan, cyclophosphamide) until the middle of 2011, and reduce intensity regimens were applied thereafter. Fanconi anemia patients received fludarabine, cyclophosphamide with or without ATG. In haploidentical hematopoietic stem cell transplantation (HHCT) before year 2016 a combination of fludarabine, cyclophosphamide and ATG was mostly used, while uniformed combination of fludarabine, thiotepa, treosulfan and ATG was used since 2016. Grafts for HHSCT were manipulated by ex vivo CD3 or αβ+ T-cell depletion.
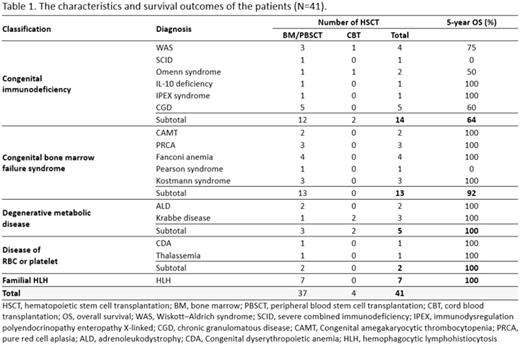
Results : This study included 14 patients with congenital immunodeficiency, 13 with congenital bone marrow failure syndrome, 5 with degenerative metabolic disease and 7 with familial hemophagocytic lymphohiotiocytosis (Table 1). By donor types, 8 patients received grafts from matched familial donor (MFD), 22 from matched unrelated donor (MUD), and 10 from haploidentical family donor(HFD), respectively.
The median age at HSCT was 2.5 years (range, 0.65-13.5 years). Grafts for HHCTs were CD3-depleted in 2 patients and αβ+ T-cell depleted in 8. The median infused dose of CD34+ cells were 9.38 x 106/kg (range, 0.15-44.11). Among 41 patients, 39 patients reached neutrophil engraftment, with a median of 11 days (range, 9-21) with no significant difference in neutrophil engraftment between donor types (P value 0.355).
Graft failure (GF) occurred in 4 patients (primary in 3 patients; Omenn syndrome, Kostmann syndrome, Thalassemia and secondary in 1 patient; Wiskott-Aldrich syndrome). One expired due to sepsis, and 1 patient diagnosed with thalassemia is alive with occasional red blood cell transfusion on an outpatient basis. The other two underwent second HHCT; one with secondary GF died due to pneumonia after engraftment, and the other experienced GF but alive with supportive care.
Mixed chimerism was detected in 24 patients 1 month after HSCT (range, 1 - 100%) and in 10 patients 1 year after HSCT (range, 1 - 80%). Grade II-IV acute GVHD occurred in 12 patients (29%, 5 with Gr II, 5 with Gr III and 2 with Gr IV), while chronic GVHD occurred in 4 patients (10%, 1 MFD, 3 MUD and all extensive). There were 4 patients who developed cytomegalovirus (CMV) disease, and 2 with sinusoidal obstruction syndrome (SOS), but no post-transplant lymphoproliferative disorder. There was no mortality related to GVHD, CMV or SOS.
As for survival results, a total of 6 patients died; one HFD recipient died of pneumonia on day +216 of second HHCT, 3 MUD recipients died of pulmonary hemorrhage and the other 1 MUD recipient died of sepsis. One MUD patient died due to sudden cardiac arrest 3 years after HSCT. The overall transplant-related mortality was 14.6%. At a median follow up 56 months (range, 0.3 - 182 months), the 5-year overall survival (OS) and event free survival (EFS) was 85.4% and 82.9%, respectively. There was no statistical difference in OS and EFS among HSCTs from 3 different donor types (OS - MFD : 100% vs MUD : 72.7% vs HFD : 90.0%, P=0.336, EFS-MFD : 100% vs MUD : 72.7% vs HFD : 80.0%, P=0.367). Patients who received HSCT at younger age (<500 days) had a worse outcome compared to the older age group (OS : 54.5% vs 96.7%, P=0.001, EFS : 63.6% vs 90.0%, P=0.038). Patients in the congenital immunodeficiency group tended to have a lower OS, although this showed no statistically significant difference (P=0.074).
Conclusion : In this study, we presented a single center experience of HSCT in pediatric rare non-malignant diseases and showed that HHCT donors were comparable to HSCT from other donor types, with no statistically significant difference in neutrophil engraftment, OS and EFS. Since an HLA haploidentical relative is available in almost every patient, the improvement of this treatment modality will offer a better chance of cure in patients with a variety of pediatric rare non-malignant diseases.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal